French remote cancer patient monitoring platform Resilience has raised $25m in a round led by existing investor Picus Capital and new backer Red River West.
VCs Cathay Innovation, Singular and Seaya Ventures — another previous investor — also participated.
The round comes six months after Resilience’s platform — which collects data from cancer patients remotely via questionnaires and allows medical professionals to monitor them — was approved for reimbursement in France, meaning patients can access it for free and the state foots the bill.
According to cofounder and CEO Jonathan Benhamou, it’s the first remote cancer patient monitoring platform in the world to be approved for reimbursement.
The fresh funds will see Resilience expand from its current markets of France and Belgium into Germany. The startup also wants to build a clinical trial platform for pharmaceutical companies.
Remote patient monitoring
Across the globe, rising cancer cases and an understaffed workforce are increasingly putting oncology departments and clinics under pressure.
Benhamou says that Resilience’s remote patient monitoring (RPM) platform — which, alongside collecting patient data, also features educational resources like articles, podcasts and videos to help patients understand and adhere to their treatment — can help tackle that problem.
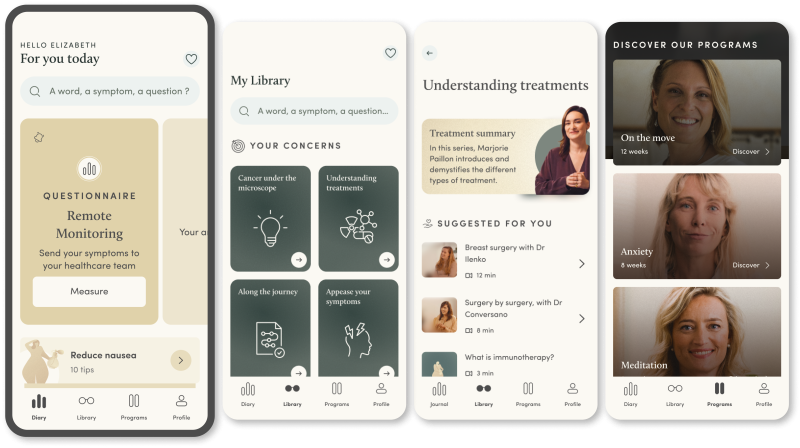
The amount of time physicians spend with patients is going down, he adds, and because of that patients don’t understand the treatments and side effects and are dropping off treatment pathways.
“It’s a huge problem. It’s a vicious cycle because if you drop off [a course of treatment] and then your drug is not efficient, then you have to go back to the hospital and start another treatment,” says Benhamou.
Resilience can help patients know what to expect, he says. “With certain drugs, we can say we know you’re going to have three days of nausea, and by saying that patients are much better able to support themselves.”
While Resilience doesn’t yet have data on how many more cancer patients its platform allows each doctor to see, Benhamou tells Sifted that results from clinical trials show how the startup’s platform “impacts the resources allocated for each patient’s care”.
According to one clinical study of 559 patients using Resilience’s platform, they saw a 6.9% reduction in emergency room visits, spent 1.62 fewer days in hospital and experienced 9.3% less severe treatment-related toxicities. Another clinical study found that patients using Resilience’s platform lived 5.2 months longer than those who didn’t.
Using a proprietary algorithm, Resilience’s platform can also flag to patients when they need to attend hospital and alert medical professionals if something’s wrong. It’s deployed in more than 90 medical centres with 10k patients in France and Belgium.
Expansion plans
In November 2023, less than three years after launch by Benhamou and his cofounder Céline Lazorthes, the company was approved for reimbursement in France — meaning doctors can prescribe the app to patients alongside cancer drugs. It’s also active in Belgium, where the startup submitted an application for reimbursement in January this year, and is awaiting approval.
Resilience began to make its first revenue in January this year, getting paid €73.33 by the French state per patient per month. It had been keeping the lights on through its years of zero revenue with €45m-worth of venture capital. Its angel investors include French billionaire Xavier Niel, Station F’s Roxanne Varza and health insurtech unicorn Alan’s CEO and cofounder Jean-Charles Samuelian.
It now plans to expand internationally — with Germany the next stop. While RPM apps aren’t yet approved for reimbursement in the country, Resilience is currently negotiating with German medical regulator BfArM about their introduction to its reimbursement scheme.
Since 2019, DiGA regulation has allowed doctors in the country to prescribe app and web-based therapy via public healthcare.
Benhamou hopes to have launched in the country by the start of 2025. “We know that RPM will be part of DiGA at some point. If [BfArM] don’t approve it for reimbursement, we will go in another way, like private insurance companies or via direct licences to hospitals.”
Geographic expansion isn’t the only thing on Resilience’s growth map. It’s also expanding the type of care it provides; in November 2023, it acquired French IBS monitoring platform GutyCare.
Selling to pharma
If everything goes to plan, most of Resilience’s revenue won’t come from governments — but from pharma companies.
In September 2023, the startup launched an RPM platform which helps pharma companies monitor patients in clinical trials.
“If you look at pharma and the contract research organisation (CRO — a company that runs clinical trials for pharma), the industries haven’t moved in years,” Benhamou says.
“When you run clinical trials, you have to collect the data on the toxicity of a drug.” Most of the time, that data is still collected on paper, Benhamou adds. “We have experience in collecting data, and we want to provide this to pharmaceutical companies.”
He thinks Resilience can digitise this process — and points to data the company has collected that shows 90% of patients that use the platform fill out the questionnaires it sends each week.
The clinical trial market size was estimated to be worth $58bn in 2023, and is set to nearly double in value by 2032. It’s a huge market, worth a quarter of the global cancer care market — which includes things like drug and treatment costs.
Resilience has four pharmaceutical customers for this product — although it’s unable to share who they are.
Read the orginal article: https://sifted.eu/articles/resilience-cancer-health-raise-news/


